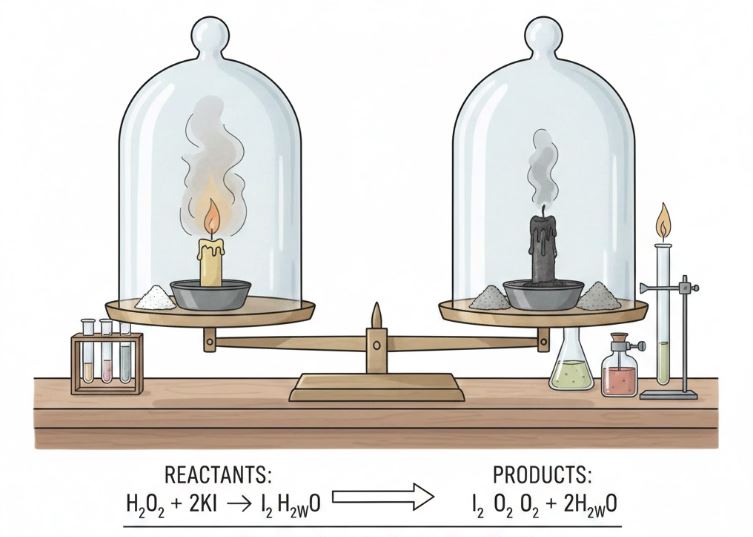
One of the principles’ of chemistry as well as classical physics is the Law of Conservation of Mass. Simply put, it states that, in a closed (isolated) system mass can neither be created nor destroyed – it is constant throughout time.
Practically this amounts to the fact that, in the case of a chemical change of some physical transformation, as, for instance, in the combustion of wood, in the solution of a salt in water, or in the melting of ice, the weight of the entire mass of substances present, thus measured, remains unchanged. The constituents can change shape or can be rearranged, however, the overall mass is held by conservation of mass.
Historical origins: from alchemy to modern chemistry
The concept of mass conservation reaches back some distance though it was not until the late 18th century that it was strongly defined as a law, owing to the efforts of the French chemist Antoine Lavoisier. In 1789, Lavoisier through his careful experiment discovered how mass was constant in a chemical reaction, including gases.
Most chemical phenomena prior to the work of Lavoisier could be attributed with mystical or qualitative explanations (e.g., the conception of phlogiston, a substance supposedly released during combustion). However, through weighing matter pre-reaction and post-reaction Lavoisier was able to scientifically demonstrate that mass was conserved. This change made chemistry a quantitative and predictive science, rather than being a hypothetical art (alchemy).
It is due to this fact that the conservation of mass law is one of the building blocks of classical chemistry, all of the balancing chemical equations to the yields of industrial chemical reactions is founded on this concept.
How it works: closed systems, Reactants & products
The law is best well established in case of a closed system – i.e. no mass is added or removed out of the system in the process. In this case, any physical transformation or chemical reaction must be subject to mass conservation.
Chemical Reactions
In a normal chemical reaction – the rearrangement of atoms gives new molecules (products) out of initial molecules (reactants). The quantity (and type) of any particular atom will be the same before and after – thus, were you to count the total number of atoms (or, more simply, add up the total mass), you will get the same result.
As an illustration, one can take the burning of methane (CH 4 ). To manage the chemical equation:
CH₄ + 2 O₂ → CO₂ + 2 H₂O
The right (products) has equal numbers of atoms, carbon, hydrogen and oxygen as the reactants (1, 4, and 4 respectively) on the left – although redistributed into carbon dioxide and water molecules’. Assuming that the gases do not escape now, the mass would be the same as you determined it before and after the sealed container.
This is what makes the difference between chemists balancing chemical equations and matching atoms representing them symbolically, they are making sure that mass is preserved. Balanced equations are equations of mass conservation.
How to Teach Conversation of Mass – Click to Explore Tips
Physical Changes
Conservation of mass is also true in the case where the matter alters state – such as in the melting of ice to water, or evaporating water to vapor, as long as the system is closed. The mass does not change but the shape does.
An example: in case of melting a 100 gram block of ice in a container that is closed, the resulting water will weigh 100 gram as well. On the same note when evaporating water is contained, the amount of water (liquid and vapor) is the same.
Everyday examples: why this law matters
- Lighting a candle or wood in a closed jar: It is a common misconception among many students to think the wax is disappearing when a candle burns since all you see is the fading wax. However, based on the law of conservation of mass – the total mass will not change when we add the gas produced (CO 2, water vapor) and smoke/ash.
- Dissolving salt in water: In case you put water and dissolved salt in a closed container, there is no change in the mass of the whole system (water plus salt). The ionized salts are simply dispersed in water yet the mass remains the same.
- Industrial chemical production: In industries that produce chemicals, the law permits the accurate computation of the yield of product that they can obtain in terms of quantity based on a known amount of reactants, which is crucial in efficiency and safety.
- Environmental and ecological research: Mass-balance techniques’ (based on the conservation law) are used by scientists to trace the path through which elements circulate in ecosystems (air, water, biomass, sediments, etc.).
Limits and modern perspective
Although the law of conservation of mass is fundamental, modern physics has improved or even replaced it in some instances.
- Equivalent Relativity and Mass Energy: The theory of relativity (E = mc 2 ) by Albert Einstein holds that mass and energy are equivalent. This is to state that in circumstances like matter being changed to energy (or the other way around), the traditional concept of the mass being conserved may fail.
- Nuclear reactions, particle physics, high-energy processes: In nuclear fission, fusion or particle-antiparticle annihilation, there need not be conservation of rest mass, some may be changed to energy (radiation), or mass may emerge out of energy.
- Open systems: The real world is hardly the case of pure isolation. Matter or energy may be added or lost to a system (gases escaping, heat exchange, etc.) making mass-balance tricky. In these situations inputs/outputs (mass/energy flows) should be taken into consideration or more general conservation laws (mass-energy, energy, momentum, etc.) should be considered.
Consequently, the Law of Conservation of Mass is very useful and correct in normal (non-quantum, non-nuclear, non-relativistic) situations (chemistry, biology, most engineering, fluid mechanics) but it is known to be approximated when quantum, nuclear or relativistic effects are involved.
Why the law is important – more than just chemistry
- Foundation of Stoichiometry and Chemical Calculations.
The absence of the conservation principle meant that an individual could never be sure of the extent of product that would be produced during a chemical reaction, or the quantity of reactant needed. Calculations of balanced equations and reactions yield are based on this law.
- The shift of Alchemy to Chemistry.
The legislation is when chemistry became a quantitative science which was no longer a mystical speculation, a thing that could be measured and predicted. The work of Lavoisier was the foundation of modern chemical science.
- Mass Balance in Environmental Science and in Engineering.
Mass balance is a method used by engineers to design processes (chemical plants, wastewater treatment, combustion engines). The same concept is used by ecologists to monitor the cycles of elements in nature (carbon, nitrogen, water).
- Conceptual Clarity: Matter Is not Magical
The philosophy and the physical truth that the law imparts is that matter does not suddenly appear or disappear, it merely transforms, or alters its configuration. This influences the most basic thinking (why setting something on fire does not cause it to vanish) up to policy (measuring pollutants, waste disposal) towards science (protective regulations in physics).
Important takeaways – What You need to know.
- According to the Law of Conservation of Mass the mass does not change in a closed system – matter is neither created nor destroyed.
- This law applies to chemical reactions’ and physical changes (melting, evaporation, combustion): the sum of the mass of all the reactants = the sum of the mass of all the products.
- Modern chemistry became a specific science that became possible due to the law and made stoichiometry and mass-balance calculable.
- However – in situations of nuclear reaction, particle physics, or great velocity, (relativity), or of open systems in which mass/energy may cross boundaries, the simple classical law is not always true. We usually think in those instances of more general conservation laws (mass-energy, energy, etc.).
Conclusion
The Law of Conservation of Mass might appear initially to be easy, mass never lost, gained, but the implications are far-reaching. It forms one of the fundamental principles upon which much of chemistry, much of physics and much of the applications of the real world, such as in the manufacture of chemicals industry and environmental science, are built.
Chemistry became a serious predictive science with the help of the visionary scientists like Lavoisier who made chemistry come out of the magical art alchemy. Although it is nowadays ameliorated with advanced physics, the law is still a fundamental conceptual tool, which informs us that the mass does not disappear when the substances change, it simply changes or reforms.
It may be that you are balancing a reaction in a chemistry laboratory or contemplating setting fire to wood, dissolving sugar, or studying an ecosystem – but remembering that mass comes in is mass out gives you a useful set of lenses to observe the behaviour of matter.